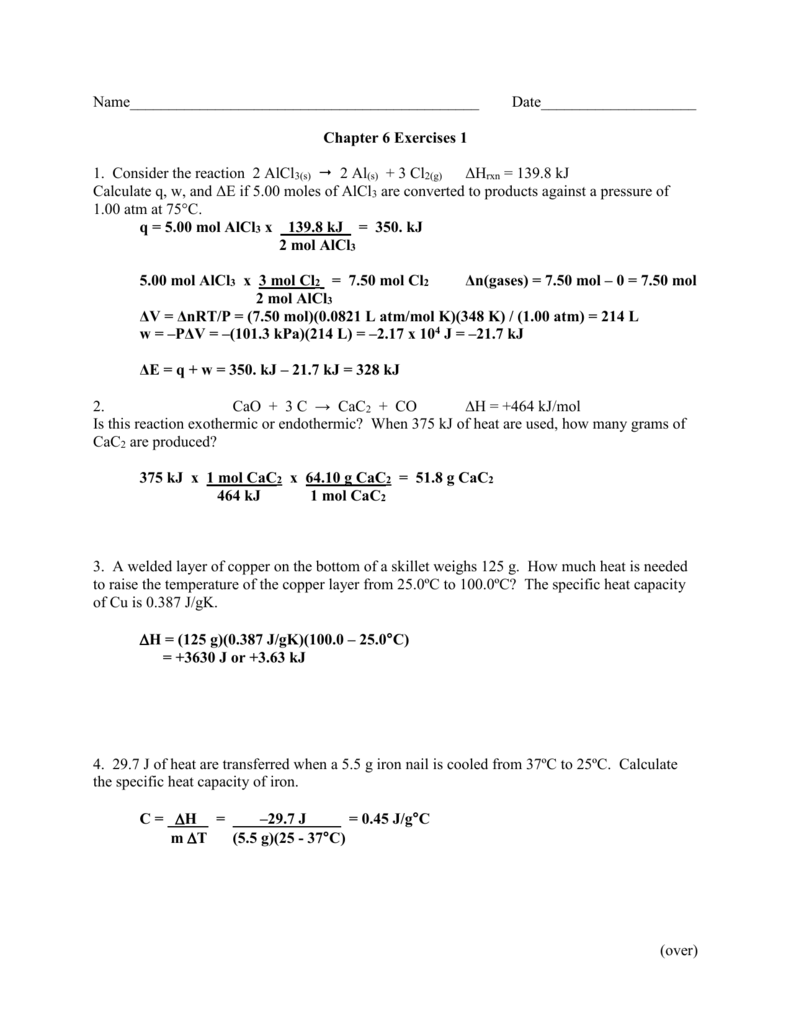
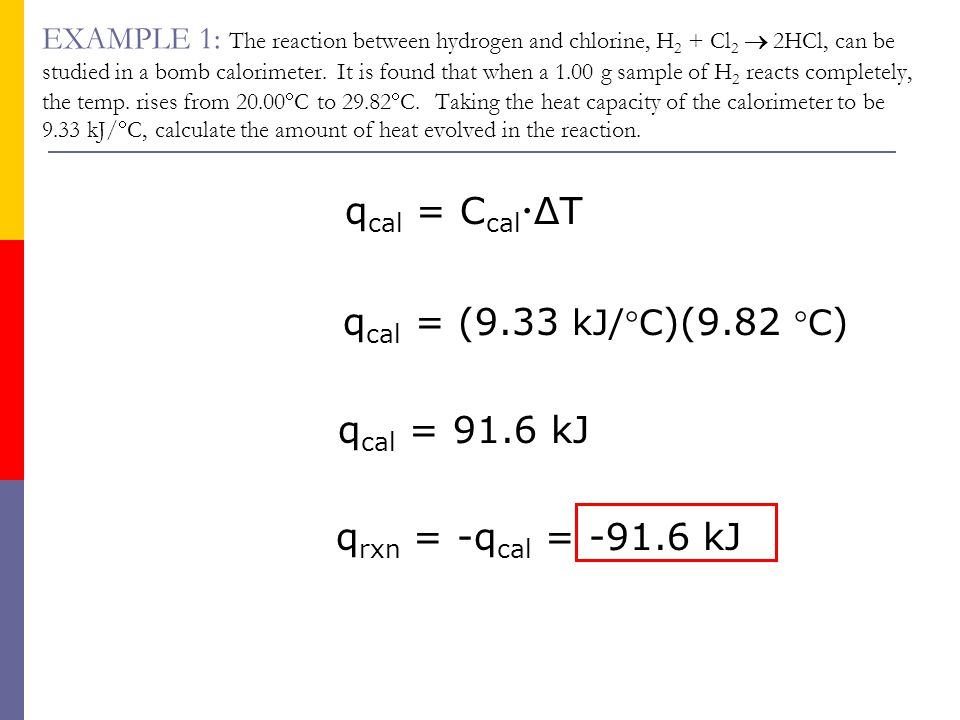
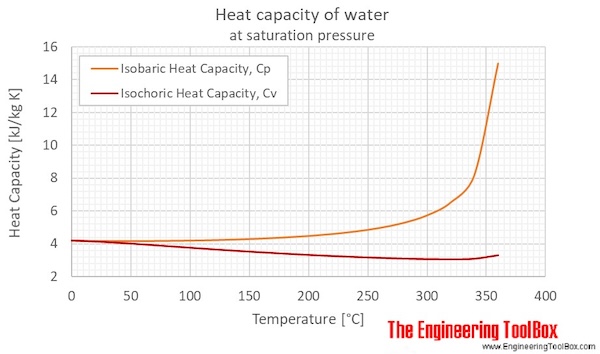
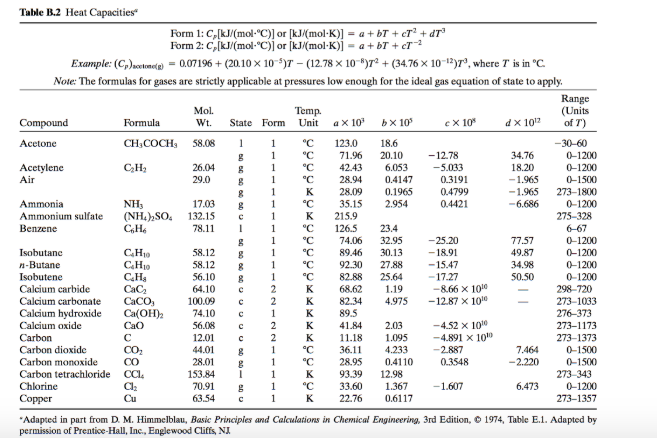
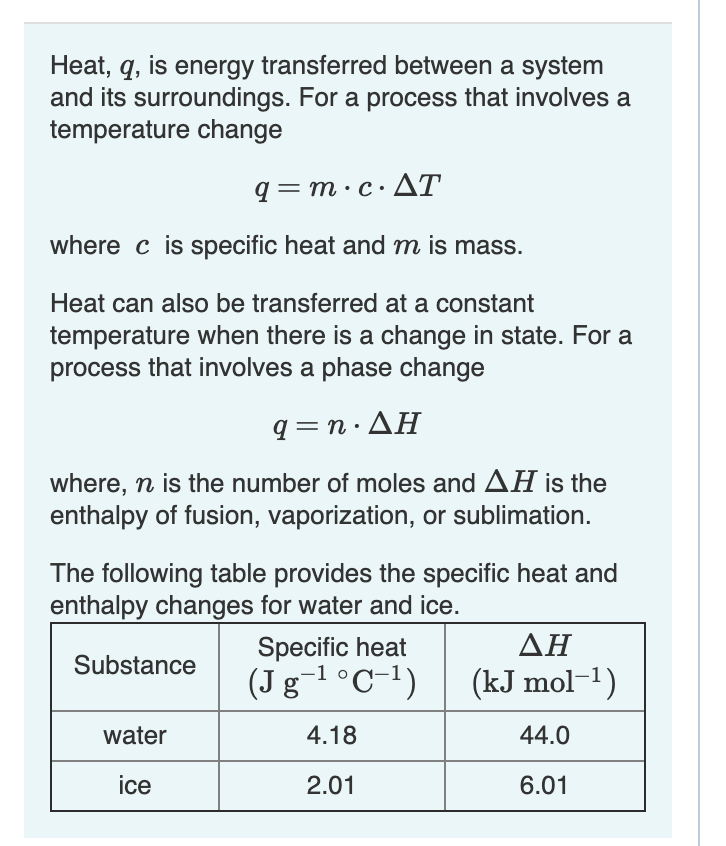
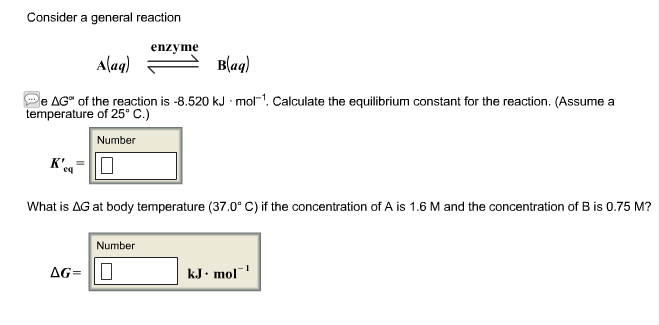
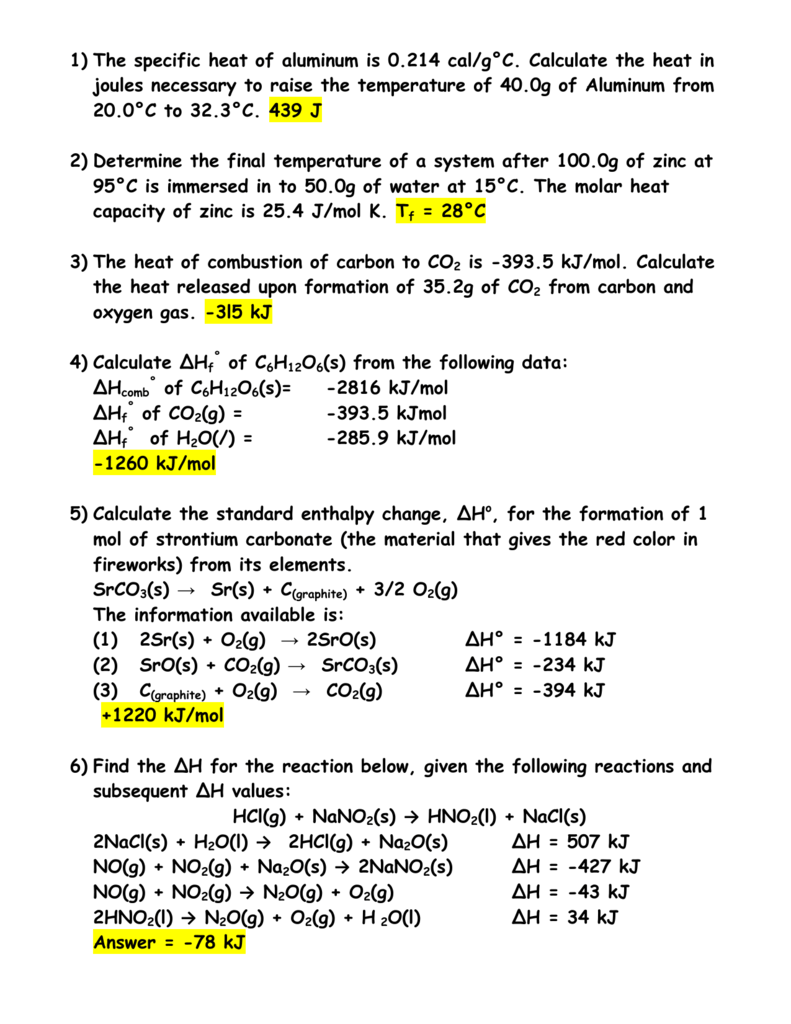
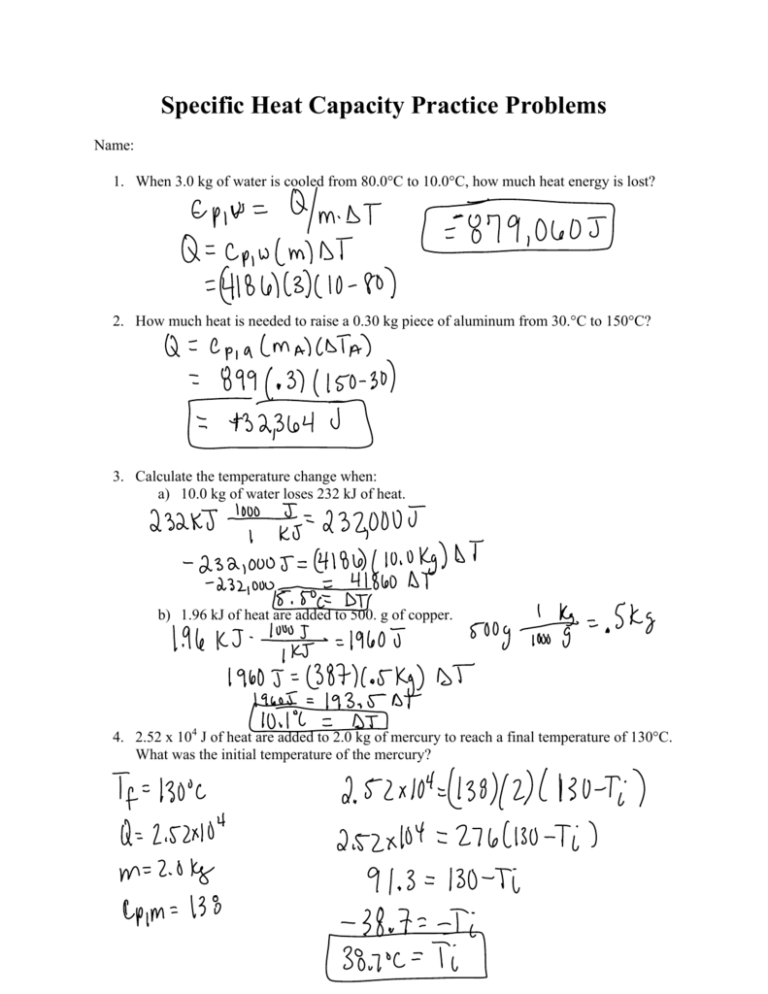
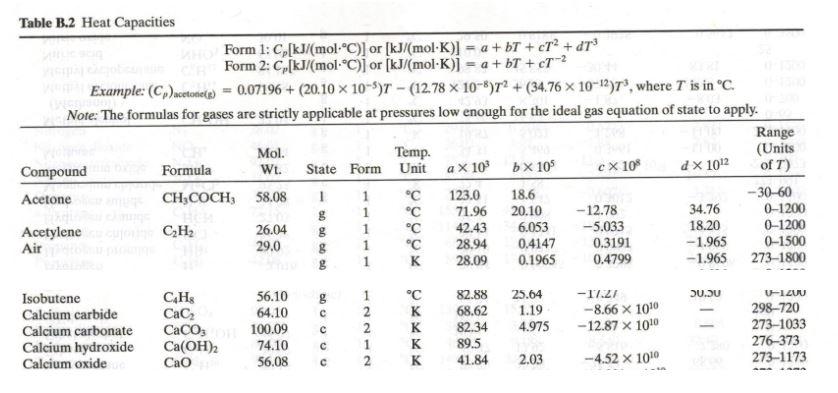
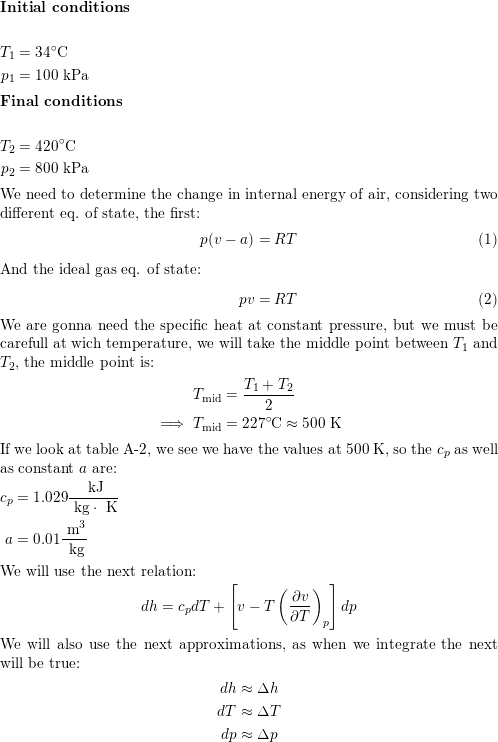SOLVED:Ethanol (C2 Hs OH) melts at _ 114 C and boils at 78 C. The enthalpy of fusion of ethanol is 5.02 kJ /mol, and its enthalpy of vaporization is 38.56 kJ /

26. For a reaction A+B - C. AH = 30 kJ mol and AS = 60 JK-mol-'. The temperature, above which reaction will be spontaneous is (1) 500°C (2) 227"C (3) 127°C (4) 175°C

RIDGID KJ-2200-C (63882) Water Jetter with Pulse, 2200 psi Working Pressure, H-30 Cart, NPT Nozzles, Foot Valve, 75 ft. Trap Hose, 110 ft. Jet Hose - at the Test Equipment Depot

USB C Tester,KJ-KayJI 2 in 1 Type C USB Tester Color Screen IPS Digital Multimeter(2021),Voltage,Current,Power,Temperature,Capacity Detector,with Clip Cable Support PD2.0/PD3.0,QC2.0/QC3.0,BC1.2: Amazon.com: Tools & Home Improvement
Solved] 1. An engine gains 550 KJ of heat from a hot source at 700 deg. celsius and rejected at 50 deg. celsius. What is the amount of the power out... | Course Hero